The Journey to Discovering Elements: A Historical Perspective
Written on
The Evolution of Element Discovery
The quest to identify chemical elements was certainly a formidable challenge for early chemists. Imagine attempting to conduct experiments on a substance that eludes visibility, possessing no discernible taste, odor, or form. While modern scientists might find it relatively straightforward to conceptualize such an experiment, it was nearly impossible for a 17th-century chemist bound by ancient theories of matter. At that time, air was still viewed as an indivisible element, considered to be in its most unrefined state. Delving into the nature of air must have seemed like a fruitless endeavor, akin to a tedious academic assignment handed to an unsuspecting student in the 18th century.
These pioneering experiments laid the groundwork for chemistry, earning a distinct label: “The Pneumatic Experiments.” This term seems to bear the hallmark of British origin, given that the prominent figures behind the work—Henry Cavendish, Joseph Priestley, and Joseph Black—were all British. While the terminology might sound a bit overly formal, it accurately captures the essence of their groundbreaking work.
Now, let’s delve into the scientific apparatus employed for these air experiments, specifically the method known as “collecting gas over water.” For those without a scientific background, operating this apparatus might not be immediately intuitive. Reflecting on my own experience as a college instructor, I remember guiding students through a challenging experiment involving the measurement of gas dissolved in a commercial beverage. The most effective approach to answering the question involved creating an apparatus to collect gas over water, but we left that discovery to the students to foster their problem-solving skills. Observing their struggle to achieve that “aha” moment was both enlightening and humbling.
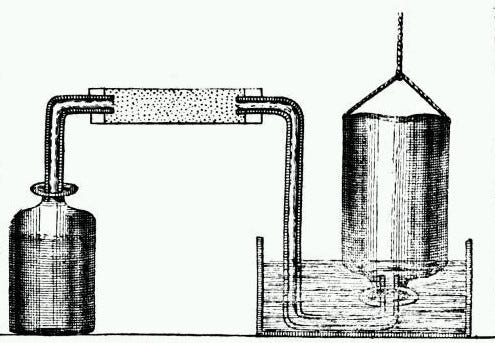
How to Collect Gas Over Water (This setup was used to discover hydrogen)
Moving through the components of this apparatus from left to right, we see a reaction vessel generating gas, which travels through a pipe into another container. Occasionally, a reagent is introduced into the pipe to purify the gas before it is collected. The vessel on the right is filled with water, and when gas is introduced, it displaces the water within. During the early 18th century, various designs existed, but they all operated on the same basic principle of collecting gas by displacing liquid. Much like messaging apps today, which share a common purpose of communication yet have distinct features.
At this time, the term "gas" was relatively novel; prior to this, all gaseous substances were simply labeled as air. In his attempts to study different types of "airs," chemist Johann von Helmont frequently broke his collection vessels and thus coined the term "gas" from the Greek word for chaos. Though it took time for the term to gain traction, the pneumatic chemists initially referred to their gases as “airs,” such as “dephlogisticated air.”
Section 1.1: The Pioneers of Pneumatic Chemistry
During this era, educational opportunities were scarce, particularly for those from lower socioeconomic backgrounds. Many individuals relied on the church for basic literacy skills. Thus, several preachers transitioned into the first practicing scientists. This was the case for Joseph Priestley, an early practitioner of pneumatic experimentation. Starting life as the son of a tailor, Priestley received his education through religious instruction before becoming a teacher. His dedication to writing and collaboration with other scientists eventually led to his sponsorship.

Joseph Priestley: The First Pneumatic Chemist
This sponsorship, often provided by affluent noblemen, allowed aspiring scientists like Priestley to conduct research without the burden of financial constraints. These sponsors, recognizing the potential of chemistry for future industrial applications, invested in the budding field. Consequently, this support afforded Priestley the opportunity to dedicate himself entirely to his research, leading to significant contributions to the study of “airs.”
Despite his enthusiasm and inventive spirit in experimentation, Priestley’s approach was somewhat chaotic. In August of 1774, however, he made a discovery that would prove monumental in the history of chemistry.
Section 1.2: The Discovery of Oxygen
Priestley’s pivotal experiment involved heating “red calx of mercury” (mercury oxide) in the presence of air and collecting the resultant gas over water. This series of experiments effectively marked the modern methodology for isolating pure elements. The term “red calx of mercury” refers to mercury oxide, which is generated when mercury undergoes calcination. Notably, Priestley modified his gas collection apparatus by substituting water with mercury, believing it to be inert with respect to gases. Through this process, oxygen was liberated, along with vaporized mercury, ultimately resulting in the collection of pure oxygen.
To witness a modern replication of Priestley’s groundbreaking experiment, check out the video below:
While Priestley did not refer to the gas as oxygen, he labeled it “dephlogisticated air,” based on the then-prevalent theory of phlogiston. According to this theory, combustion was attributed to the transfer of phlogiston from substances to air. A limited quantity of air could only accommodate a certain amount of phlogiston; thus, when a candle extinguished in a sealed container, it was due to the air being saturated with phlogiston. Conversely, dephlogisticated air was considered to be devoid of phlogiston, ready to engage with whatever was presented.
This understanding, though flawed by modern standards, was a significant step forward. Oxygen’s role in combustion was recognized, yet the notion of phlogiston persisted and would later be contested as chemistry evolved. The eventual naming of oxygen would come later, attributed to another figure in the scientific community.
With this landmark discovery, chemistry began to take its initial steps toward becoming a recognized scientific discipline. However, the lingering influence of phlogiston would present challenges that early chemists would need to navigate as they advanced their understanding. Further critical investigations into “airs” would continue, which we will explore in subsequent discussions.
